Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - How To Classify Chemical Reactions For Ap Chemistry Albert Io : Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - How To Classify Chemical Reactions For Ap Chemistry Albert Io : Learn vocabulary, terms, and more with flashcards, games, and other study tools.. For details on it (including licensing), click here. This is types of chemical reactions: Scientists classify them based on what happens when going from reactants to products. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations.
Classify each of these reactions. For details on it (including licensing), click here. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Transcribed image text from this question. Reactions that can be classified as double displacements include precipitation reactions, neutralization in this lab you.

Synthesis, decomposition, single and double replacement.
Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. This is types of chemical reactions: Sn + 2 br, snbr. This chemistry video tutorial discusses the different types of chemical reactions that you need to know such as combination reactions, synthesis and decompos. Synthesis, decomposition, single and double replacement. This worksheet would work well with a chemistry class. What is this type of chemical reaction called? Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Synthesis combustion decomposition single replacement. Redox reactions therefore include combustion reactions, single displacement reactions, and most. This chemistry video tutorial explains how to classify different types of chemical.
2 hbr + sn snbr; Synthesis combustion decomposition single replacement. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. S + o2 → so2 cacl2 + 2agno3 → ca(no3)2 + 2agcl. What types of reactants are usually involved in synthesis, decomposition, single displacement, and double displacement reactions?
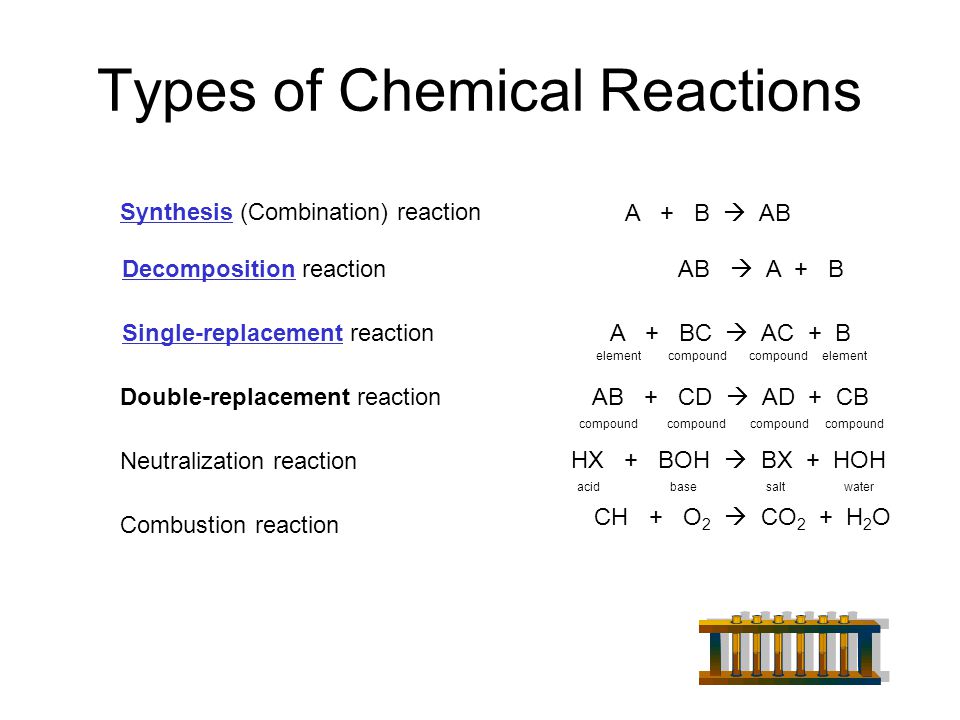
H2 + cl2 2hcl synthesis ca + 2h2o ca(oh)2 + h2 single displacement practice 2co + o2 2co2 synthesis 2kclo3 2kcl + 3o2 decomposition practice ch3sh + 3o2 co2 + 2h2o + so2 combustion zn + 2hcl zncl2 + h2 single replacement predicting the outcomes of chemical reactions predicting single.
This chemistry video tutorial explains how to classify different types of chemical. Synthesis, decomposition, single replacement, double displacement type: Having a thorough understanding of these types of reactions will be. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. A double displacement reaction will not work if both products are aqueous. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. Predict the products of simple reactions. Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: / • it is the exact opposite of synthesis; Exchange or metathesis) redox v redox nano, hci nacl+ hno, combination (synthesis. Chemical equilibrium is a state of a chemical system in which several chemical reactions take place and the rates in each pair of direct. Learn vocabulary, terms, and more with flashcards, games, and other study tools. S + o2 → so2 cacl2 + 2agno3 → ca(no3)2 + 2agcl.
Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. H2 + cl2 2hcl synthesis ca + 2h2o ca(oh)2 + h2 single displacement practice 2co + o2 2co2 synthesis 2kclo3 2kcl + 3o2 decomposition practice ch3sh + 3o2 co2 + 2h2o + so2 combustion zn + 2hcl zncl2 + h2 single replacement predicting the outcomes of chemical reactions predicting single. Terms in this set (5). What types of reactants are usually involved in synthesis, decomposition, single displacement, and double displacement reactions? Synthesis, decomposition, single and double replacement.
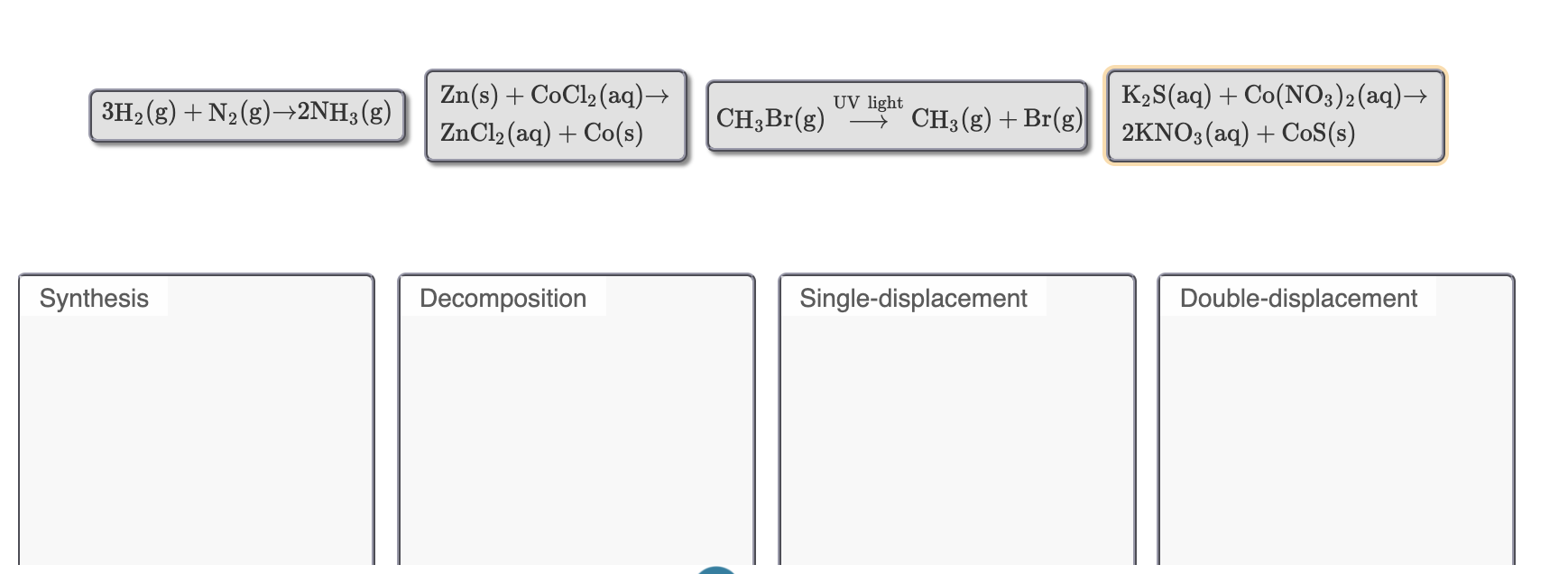
Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement.
H2 + cl2 2hcl synthesis ca + 2h2o ca(oh)2 + h2 single displacement practice 2co + o2 2co2 synthesis 2kclo3 2kcl + 3o2 decomposition practice ch3sh + 3o2 co2 + 2h2o + so2 combustion zn + 2hcl zncl2 + h2 single replacement predicting the outcomes of chemical reactions predicting single. Decomposition single replacement double replacement. Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Synthesis, decomposition, single replacement, double displacement type: This chemistry video tutorial explains how to classify different types of chemical. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Chemical equilibrium is a state of a chemical system in which several chemical reactions take place and the rates in each pair of direct. Redox reactions therefore include combustion reactions, single displacement reactions, and most. Exchange or metathesis) redox v redox nano, hci nacl+ hno, combination (synthesis. 6.) sythesis and decomposition reactions are sometimes referred to as opposite reactions. Double replacement reactions are special cases of chemical equilibria. The chemical reactions we have described are only a tiny sampling of the infinite number of chemical reactions possible. In the broader aspect, there are three types of reactions:
Komentar
Posting Komentar